Thermodynamics is a basic science that deal with energy. In other words thermodynamics may be defied as the study of energy. It’s forms and it’s transformation and interaction with matter.
Thermodynamics is concerned with the transfer of heat and work in many cases with ultimate object of converting one form of energy to another.
There are four laws of thermodynamics, which are fundamental principles that govern the behavior of energy and heat in a system. These laws describe how energy is conserved, how heat is transferred, and how work is done.
Energy
Energy may be viewed as the capacity to do work or the ability to cause changes
J = Kgm2/s2
Heat
The form of energy that is transformed between two system by virtue of temperature difference between them.
Work
Work is the transfer of energy from one object to another or the transfer of energy through a force acting over a distance. In physics, work is defined as the product of the force acting on an object and the displacement of the object in the direction of the force.
J= Nm
System
A system is defined as a particular quantity of matter or a particular region of space for study.
Surrounding
The region outside a system is called the surrounding
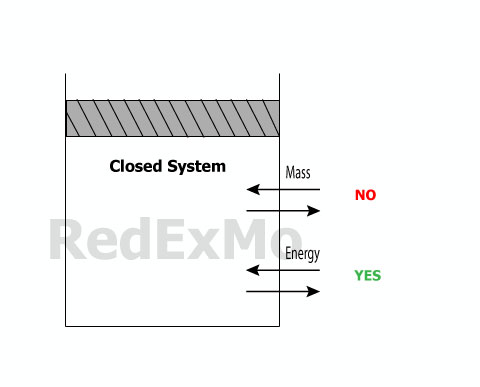
Closed System
A closed system consists of a constant amount of mass cannot cross it’s boundaries.
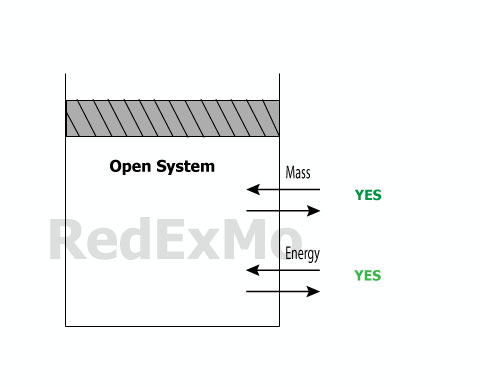
Open System
Both energy and mass can cross te boundaries of a control volume. Which is called the control surface.
Nozle, diffuser, comressure, turbine
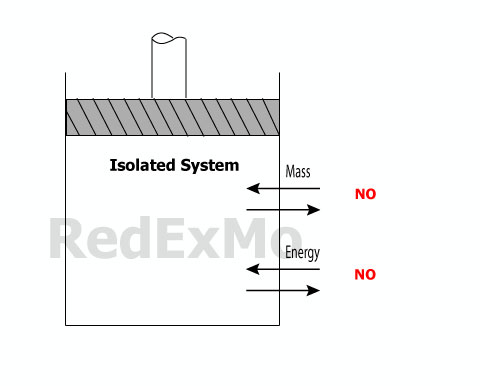
Isolated System
It is a special case of a closed systemwhere even energy is not allow to cross the boundaries.
Piston, cylinder device.
Isothermal Process
It is process during which temperature of the system remain constant
Mechanical Equilibrium
The process of the system is equal to that of the environment
Zeroth Law of Thermodynamics
If two bodies are in thermal equilibrium with a third body. They are also in thermal equilibrium with each other.
First Law of Thermodynamic
Energy can neither be created nor be destroyed. It can only change form
Or
During an interaction between a system and it’s surrounding the amount of energy gained by the system is equal to the amount of energy lost by the surrounding.
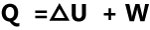
Adiabatic Process
No heat is transferred,
i.e. Q = 0 then the first law of thermodynamics is used since it’s relates W and Q
Second Law of Thermodynamics
It is impossible to construct a system which will operate in a cycle extract heat from a reservoir and do an equivalent amount of work on the surroundings.
Reversible
A reversible process is one which, when reversed reaches the initial state such that there are no changes in the environment and the rest of universe.
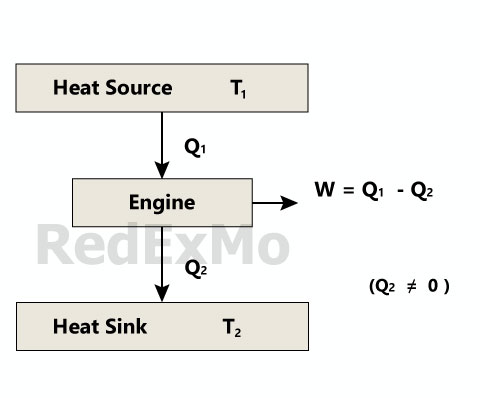
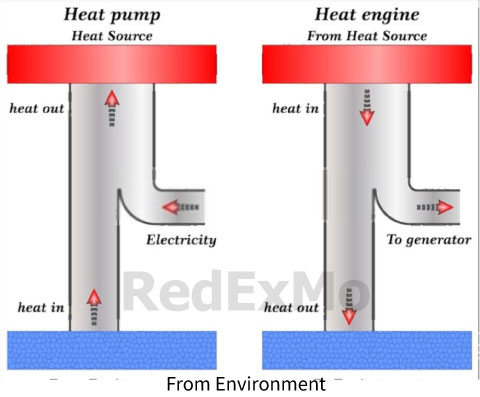
Heat Engine
A heat engine is a device that converts heat energy into mechanical work. It operates on the principle of the conversion of heat into work, which is made possible by the transfer of heat from a high-temperature body to a low-temperature body.
Heat Pump
A heat pump is a device that is used to transfer heat from one location to another. It operates on the principle of refrigeration, which involves the transfer of heat from a low-temperature body to a high-temperature body using a working fluid, such as a refrigerant.
Heat pumps are classified as either air-source or ground-source, depending on the source of the heat that is being transferred. Air-source heat pumps use the air outside as the source of heat, while ground-source heat pumps use the ground or underground water as the source of heat.
Heat pumps are used for a variety of applications, including heating and cooling buildings, heating water, and drying clothes. They are an energy-efficient alternative to traditional heating and cooling systems because they use less energy to transfer heat than is required to generate it. This is because heat pumps use a small amount of energy to move heat from one location to another, rather than generating heat through the combustion of fossil fuels.
Engine Turbocharger
A turbocharger, also known as a turbo, is a device that is used to increase the power output of an internal combustion engine by forcing more air into the engine’s combustion chamber. It does this by using the energy from the engine’s exhaust gases to drive a turbine, which in turn drives a compressor that forces more air into the engine.
Turbochargers are most commonly used on diesel and gasoline engines in vehicles, but they can also be used on other types of engines, such as aircraft engines and industrial engines. They are an effective way to increase the power output of an engine without increasing its size, which makes them popular in the automotive industry.
Turbochargers work by using the energy in the exhaust gases to spin a turbine, which is connected to a compressor by a shaft. The compressor takes in air from the atmosphere and compresses it, increasing its pressure and temperature. This compressed air is then fed into the engine’s combustion chamber, where it mixes with fuel and burns, generating power. The increased airflow provided by the turbocharger allows the engine to burn more fuel and produce more power, resulting in increased performance.
Temperature
Temperature is a measure of the average kinetic energy of the molecules in a substance. It is a measure of the degree of hotness or coldness of a substance and is usually measured in units of degrees Celsius (°C), degrees Fahrenheit (°F), or kelvins (K).
Temperature is an important physical property of matter because it affects the behavior of substances and the rates of chemical reactions. As the temperature of a substance increases, the average kinetic energy of its molecules increases, and they move faster. Conversely, as the temperature of a substance decreases, the average kinetic energy of its molecules decreases, and they move slower.
Saturation Temperature
Saturation temperature, also known as the boiling point, is the temperature at which a substance changes from a liquid to a gas at a given pressure. It is the temperature at which the vapor pressure of a liquid is equal to the atmospheric pressure.
For example, the saturation temperature of water at atmospheric pressure (1 atmosphere) is 100°C (212°F), which is the boiling point of water. At this temperature, the vapor pressure of water is equal to the atmospheric pressure, and water will boil and turn into steam. If the pressure is increased, the saturation temperature of water will also increase, and it will take a higher temperature to boil the water. Conversely, if the pressure is decreased, the saturation temperature will also decrease, and it will take a lower temperature to boil the water.
Saturation temperature is an important concept in thermodynamics because it is related to the properties of fluids and the behavior of gases. It is also a key factor in the design of many types of heat exchangers, boilers, and other equipment that involve the transfer of heat and the boiling of liquids.